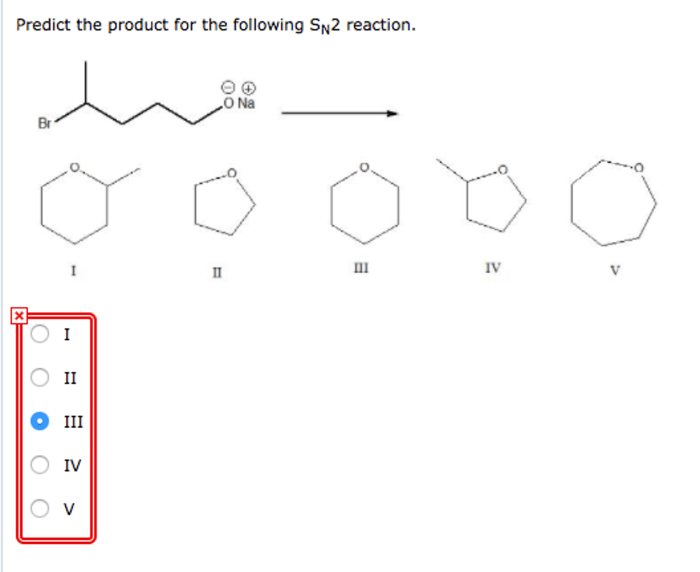
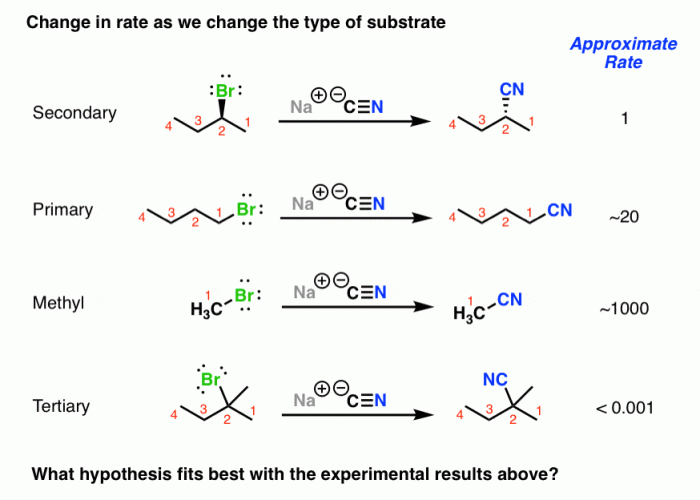
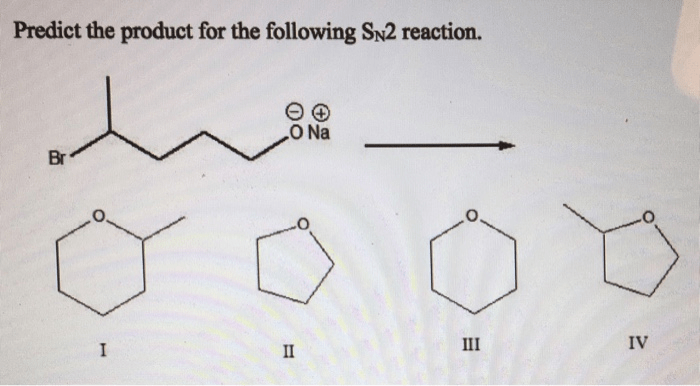
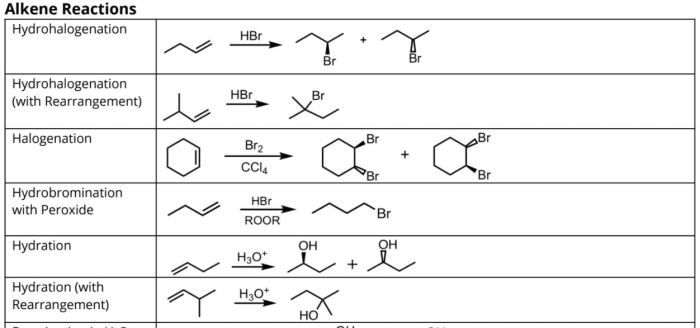
Predict the product for the sn2 reaction shown – Predicting the product of a given SN2 reaction is a crucial step in organic synthesis. This article provides a comprehensive overview of the key factors influencing product prediction, including nucleophilicity, electrophilicity, steric hindrance, and reaction mechanisms. We will also explore predictive methods and discuss practical applications of SN2 reactions.
Introduction: Predict The Product For The Sn2 Reaction Shown
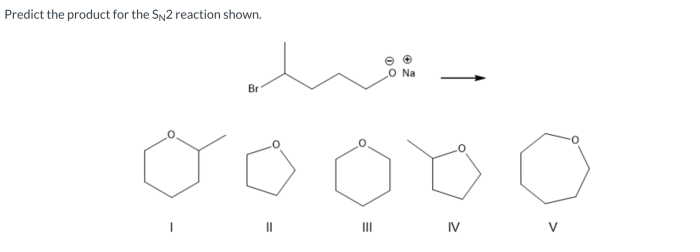
SN2 reactions, also known as nucleophilic substitution reactions, are fundamental organic reactions that involve the substitution of a leaving group by a nucleophile. These reactions are highly significant in organic synthesis and play a crucial role in the formation of various organic compounds.
Predicting the product of a given SN2 reaction is essential for designing efficient synthetic strategies and understanding the reaction mechanisms. This article aims to provide an overview of the key factors influencing product prediction in SN2 reactions.
Key Factors Influencing Product Prediction, Predict the product for the sn2 reaction shown
Several factors influence the product outcome in SN2 reactions, including:
- Nucleophilicity:Nucleophilicity refers to the ability of a species to donate electrons and attack an electrophile. Stronger nucleophiles react more readily with electrophiles, leading to faster reaction rates and higher product yields.
- Electrophilicity:Electrophilicity measures the ability of a species to accept electrons. Stronger electrophiles are more susceptible to nucleophilic attack, resulting in higher reaction rates and product formation.
- Steric Hindrance:Steric hindrance arises from the presence of bulky groups around the reaction center. Increased steric hindrance can hinder the approach of the nucleophile to the electrophile, leading to slower reaction rates and reduced product yields.
Reaction Mechanisms and Stereochemistry
SN2 reactions proceed via a concerted mechanism, where the nucleophile attacks the electrophile simultaneously as the leaving group departs. This results in an inversion of configuration at the reaction center.
The stereochemistry of the reactants plays a crucial role in determining the product formation. In SN2 reactions, the nucleophile attacks the electrophile from the opposite side of the leaving group, leading to an inversion of configuration.
Predictive Methods
Predicting the product of an SN2 reaction involves considering the factors discussed above. Several methods can be employed to aid in product prediction:
- Empirical Rules:Empirical rules, such as the “SN2 order of reactivity” for nucleophiles, provide a qualitative assessment of nucleophilicity and can be used to predict the relative reactivity of different nucleophiles.
- Computational Methods:Computational methods, such as molecular orbital theory, can provide insights into the electronic structure of reactants and transition states, aiding in the prediction of product formation.
- Kinetic and Thermodynamic Data:Kinetic and thermodynamic data, such as reaction rates and equilibrium constants, can be used to predict product selectivity and the extent of reaction.
Examples and Applications
SN2 reactions find widespread applications in organic synthesis, including:
- Alkylation of alcohols and phenols
- Acylation of alcohols and amines
- Formation of ethers and esters
Product prediction in SN2 reactions is crucial for designing efficient synthetic strategies and optimizing reaction conditions to achieve desired products.
FAQ Summary
What is an SN2 reaction?
An SN2 reaction is a type of nucleophilic substitution reaction in which a nucleophile attacks an electrophile, resulting in the inversion of the configuration at the reaction center.
What factors influence the rate of an SN2 reaction?
The rate of an SN2 reaction is influenced by the nucleophilicity of the nucleophile, the electrophilicity of the electrophile, and the steric hindrance around the reaction center.
How can I predict the product of an SN2 reaction?
The product of an SN2 reaction can be predicted using empirical rules, such as the “SN2 order of reactivity” for nucleophiles, or by using computational methods, such as molecular orbital theory.