Embark on an educational journey with our comprehensive classifying and balancing equations worksheet answers. This guide unveils the intricacies of chemical equations, empowering you to master the art of balancing chemical reactions with precision and confidence.
Delve into the fundamentals of classifying equations, unraveling the significance of balanced and unbalanced equations. Discover the step-by-step methods for classifying equations, including counting atoms and utilizing coefficients. Equip yourself with effective techniques for balancing equations, such as the half-reaction method and the oxidation-reduction method.
Grasp the underlying principles through illustrative examples.
Introduction to Classifying and Balancing Equations
Chemical equations are symbolic representations of chemical reactions that provide a concise and informative way to describe the transformation of reactants into products. Classifying equations as balanced or unbalanced is crucial for understanding the stoichiometry of reactions and ensuring that the number of atoms of each element is conserved throughout the process.
Methods for Classifying Equations
Counting Atoms
A simple method for classifying equations is to count the number of atoms of each element on both sides of the equation. If the number of atoms of each element is the same on both sides, the equation is balanced.
Otherwise, it is unbalanced.
Using Coefficients
Another method for classifying equations is to examine the coefficients in front of each chemical formula. Coefficients represent the number of moles of each reactant or product involved in the reaction. If the coefficients are whole numbers and the number of atoms of each element is the same on both sides of the equation, the equation is balanced.
Techniques for Balancing Equations
Half-Reaction Method
The half-reaction method involves dividing the overall reaction into two half-reactions, one for oxidation and one for reduction. Each half-reaction is then balanced separately before combining them to form the balanced overall equation.
Oxidation-Reduction Method
The oxidation-reduction method focuses on identifying the species that are oxidized and reduced in the reaction. The oxidation states of the atoms in the reactants and products are used to determine the number of electrons transferred and to balance the equation accordingly.
Practice Problems and Solutions
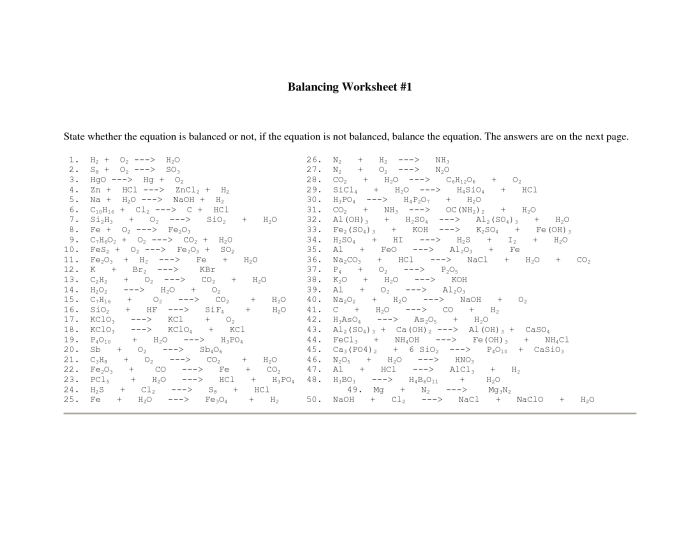
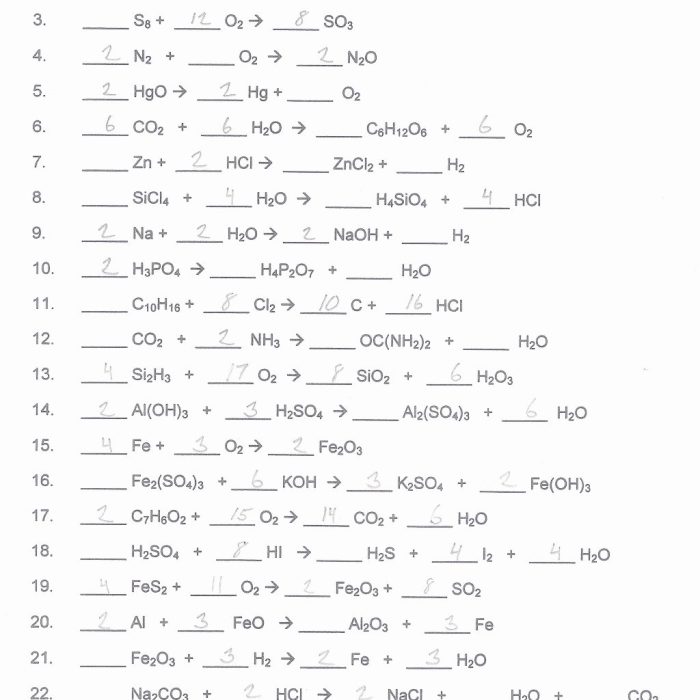
A set of practice problems involving unbalanced equations is provided. The problems are organized in order of increasing difficulty, starting with simple equations and progressing to more complex ones. A separate section for solutions is also provided, offering step-by-step explanations for each problem.
Advanced Concepts in Equation Balancing
Balancing Equations in Solution
Balancing equations in solution involves considering the presence of ions and the dissociation of electrolytes. The net ionic equation is balanced, taking into account the species that are actually present in solution.
Balancing Redox Reactions, Classifying and balancing equations worksheet answers
Balancing redox reactions requires understanding the principles of oxidation and reduction and the transfer of electrons. The half-reaction method or the oxidation-reduction method can be used to balance redox reactions.
User Queries: Classifying And Balancing Equations Worksheet Answers
What is the significance of classifying equations as balanced or unbalanced?
Classifying equations as balanced or unbalanced helps determine if the number of atoms of each element on the reactants’ side matches the number of atoms of the same element on the products’ side. Balanced equations ensure that the law of conservation of mass is upheld.
What are the key methods used for classifying equations?
Counting atoms and using coefficients are the primary methods for classifying equations. Counting atoms involves manually tallying the number of atoms of each element on both sides of the equation. Using coefficients involves analyzing the numerical values in front of chemical formulas to determine the relative proportions of reactants and products.
What are some effective techniques for balancing equations?
The half-reaction method and the oxidation-reduction method are two widely used techniques for balancing equations. The half-reaction method involves splitting the reaction into two half-reactions, one for oxidation and one for reduction. The oxidation-reduction method focuses on identifying the changes in oxidation states of atoms to balance the equation.